श्री चित्रा तिरुनाल आयुर्विज्ञान और प्रौद्योगिकी संस्थान, त्रिवेंद्रम
विज्ञान एवं प्रौद्योगिकी विभाग, भारत सरकार के अधीन एक राष्ट्रीय महत्व का संस्थान
Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum
An Institution of National Importance, Department of Science and Technology, Govt. of India





CALL FOR TECHNOLOGY TRANSFER – EXPRESSION OF INTEREST (EOI)
TiN coated coronary stent system
Intended end use : The Cobalt-Chromium-Nickel-Tungsten alloy L605 based, Titanium Nitride coated “bare metal” coronary stent system is indicated for improving coronary luminal diameter in patients with native coronary lesion length < 50 mm, and reference vessel diameter (D Ref Vessel) ranging from 2.0 mm < D Ref Vessel < 4.50 mm.
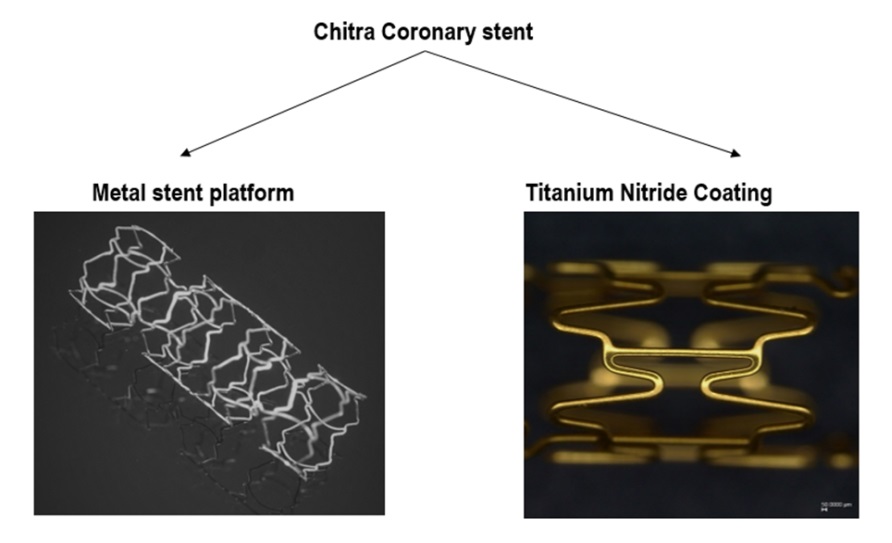
Product description : The Chitra coronary stent consists of the following components:
1. L605 based Metal Stent platform
2. Titanium Nitride Ceramic coating
Novelty(please specify patents if any filed) : Patent granted, 357096 dated 29/01/2021
Current Development stage/ Evaluations done :
# Design and choice of materials completed. 12 TiN coated coronary stent are prototyped.
# Design Verification: The safety and performance of the design has been assessed in vitro and in silico by:
(a) Stent diameter to balloon expansion characterization as per ASTM 2081-06 (2017)
(b) Dimensional verification as per ASTM F2081-06 (Reapproved 2017)
(c) Recoil study as per ASTM F2079, ISO 25539-2
(d) Foreshortening study as per ASTM 2081-06 (2017)
(e) Percent solid area study as per ASTM 2081-06 (2017)
(f) Computational structural analysis (ASTM F2514-08 Reapproved 2014)
(g) Fatigue & durability- computational analysis (ASTM F2514)
(h) Flexibility of stent as per ASTM F2606-08 (Reapproved 2014)
(i) Flexibility of stent system as per ASTM F2606-08 (Reapproved 2014)
(j) Dogboning studies as per ISO/DIS 25539-2:2019(E)
(k) Deflation time at labeled RBP as per ISO/DIS 25539-2:2019(E)
(l) Deflation time corresponding to deployed diameter as per ISO/DIS 25539-2:2019(E)
(m) TiN coating thickness measurement as per ISO/DIS 25539-2:2019(E)
(n) TiN coating surface roughness as per ISO/DIS 25539-2:2019(E)
(o) TiN coating integrity as per ISO/DIS 25539-2:2019(E)
(p) TiN coating trace element analysis as per ISO/DIS 25539-2:2019(E)
(q) DFSS based robustness (z) assessment: Critical to Quality parameters, vendor evaluation of raw materials and manufacturing process.
(r) POC Animal studies- Completed animal studies of 3 Chitra stents
The safety and performance indicators showed that the Chitra Stent is safe.
Industry responsibility for technology transfer :
# Preclinical evaluation
# Clinical trials
# Instructions For Use
# Packaging
# Regulatory
Deliverables from Institute : Technical Documentation
Work procedures & Test reports (21 Nos)
Design dossier
Technology Transfer Documentation (jointly with Industry)
# TTDOC1- Design & specifications
# TTDOC2- Raw materials and consumables
# TTDOC3- Equipment and Utilities
# TTDOC4- Jigs and fixtures
# *TTDOC5- Manufacturing process
# *TTDOC6- QA/QC